Real-Life Communication
You work as a chemical technician in a company that is doing research
and development in fuel cells. There's a big open house at the company for
potential investors.
One of the investors starts talking to you about
the fuel cells.
"You know, I failed chemistry when I was in school
and I've never really understood it since. These fuel cells sound interesting,
but I don't really see how they work. Can you explain it to me?"
So
you whip out your handy-dandy pamphlet, called Principal Operation of a Fuel
Cell, to give to him as an explanation.
He sees what you're doing and
waves his hands. "I've already seen that, and it's all Greek to me! Explain
it in plain, simple English, would you please?"
Use the following excerpt
from the pamphlet to explain how fuel cells work. Use as many diagrams as
necessary and don't be afraid to make it really simple -- remember he failed
high school chemistry!
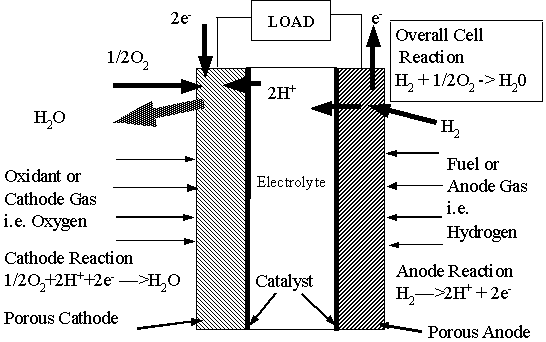
"As shown
in the typical hydrogen-oxygen fuel cell representation, Principal of Operation
of a Fuel Cell, the solid electrolyte membrane is sandwiched between two electrodes
fashioned of porous carbon. A platinum catalyst is positioned between the
carbon and the membrane. The entire assembly of membrane, electrodes and catalyst
forms one integrated unit called the Membrane Electrode Assembly (MEA). This
MEA is located between two graphite plates to form a single cell. Electrical
contact to the electrodes may be made through these fluid flow field plates.
"When
hydrogen is supplied to the anode, it diffuses through the porous electrode
to the catalyst layer. The catalyst causes the hydrogen to dissociate into
protons (H+) and electrons. The electrolyte is a proton conductor so the protons
flow through the electrolyte to the cathode. The electrolyte is an electron
insulator, and thus for the reaction to be complete the electrons must flow
through the external circuit to the cathode. At the cathode, the protons,
electrons and oxygen combine at the catalyst to form water."