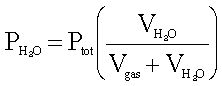Real-Life Math
You work with a high-tech company that makes fuel cells. Basically,
a fuel cell is a source of energy. It works by breaking down a fuel on an
atomic level. This creates an electrical current, and the byproduct is pure
water!
As one of the chemical technicians on staff, you are responsible
for quality control. You test the fuel cells to make sure they are working
properly.
One of the ways you make sure they are working properly is
by measuring the amount of water they produce. You know how much water should
be produced by each cell in a given time. Yet 1 of the cells you have tested
is only producing about 1/2 as much water as you expect it to.
One
of the reasons might be that there isn't enough water getting into the cell
in the first place. The water gets into the cell in the form of vapor. The
water vapor is mixed with the fuel -- in this case, hydrogen -- before it
enters the cell. The machine that mixes the water vapor with the hydrogen
is called the humidifier.
You want to double-check how much water vapor
is in the hydrogen before it enters the fuel cell. So, you go over to the
humidifier and take a reading. The humidifier's instruments tell you the volume
of water vapor in the hydrogen is 12 standard liters per minute (SLPM). But
the humidifier can be wrong -- it's only a machine! You'll want to calculate
the volume of water vapor in the hydrogen yourself.
You quickly
collect the data you need, measuring outputs like volume and pressure from
the fuel cell itself. Now, using the following formulas and the data you've
collected (shown below), determine the volume of water vapor getting into
the cell. Compare your answer with the humidifier's reading. Is it the same
or different? Is the humidifier working properly?
Here is the first
formula you will need:
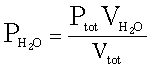
Where:
PH20
= pressure of the water vapor in the system
Ptot = total pressure
of the system
VH20 = volume of water in hydrogen
Vtot
= total volume of hydrogen and water
Here is the second
formula you will need:
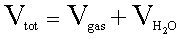
Here
is your data:
The pressure of the water vapor in the system
is 7.1 PSIA
(PSIA stands for "pounds per square inch absolute")
The
total pressure of the system is 44.7 PSIA
The volume of gas (hydrogen)
is 28 SLPM
(SLPM stands for "standard liters per minute")
Here's
a hint: